Gene Details: ALG5
1 / 1
General Information
Gene Name: ALG5 (Dolichyl-phosphate beta-glucosyltransferase)
Synonym:
Short Names: DolP-glucosyltransferase;
Alternative Names: Asparagine-linked glycosylation protein 5 homolog;
Notes:
- Involved in the synthesis of Dol-P-Glc, using UDP-Glc as donor.
Description from Dr.Glyco-GPT:
Warning: LLMs can generate factually incorrect information, as they simply predict the next word based on training data. Always verify LLM output by cross-checking with reliable sources!
Catalytic Activity
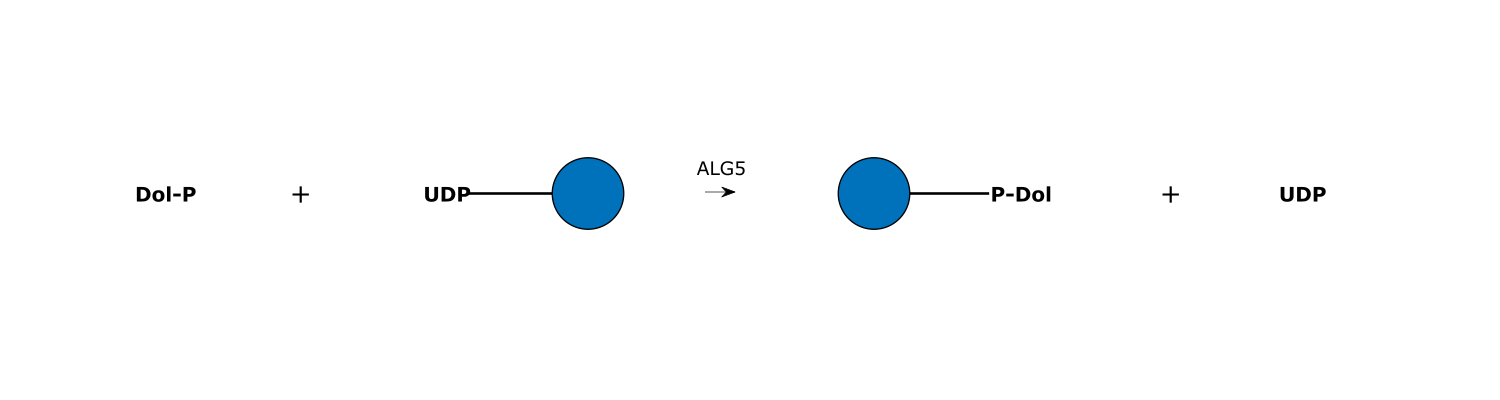
Reaction and Disease Links
EC # (IUBMB):
2.4.1.117
KEGG: 29880
Reactome :
R-HSA-480985
Transcript levels (Cell lines and Single cell data) URL
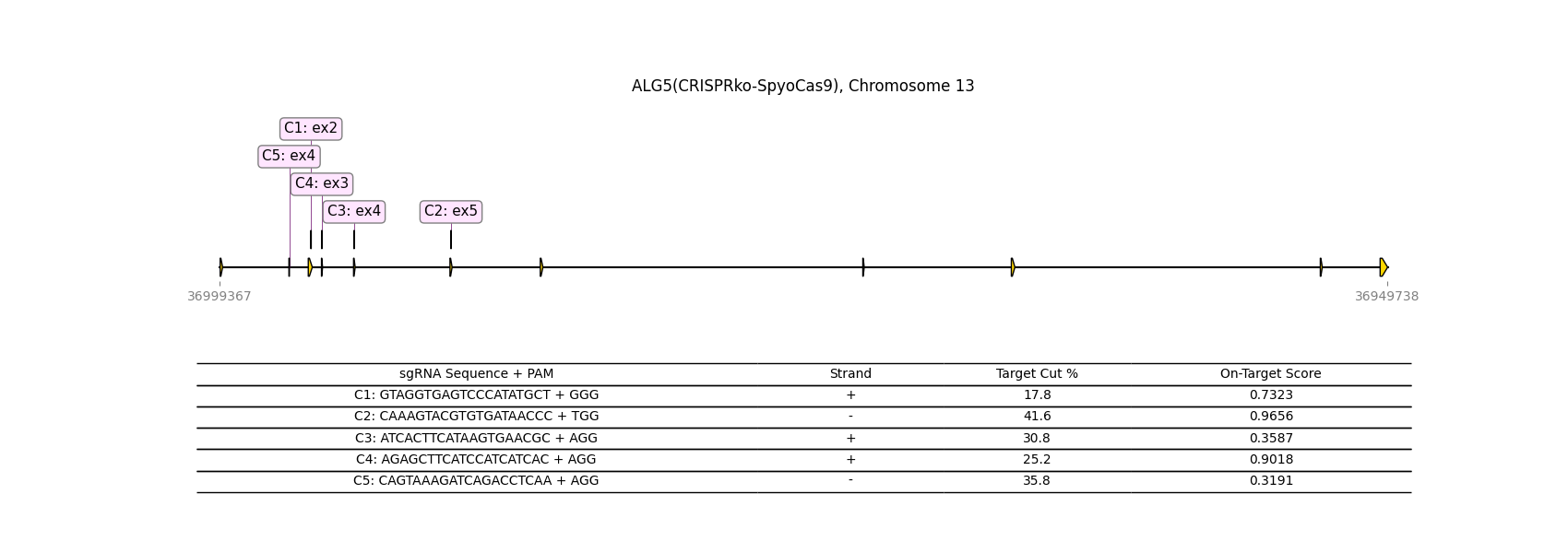
CRISPR-knockout
CRISPR-activation

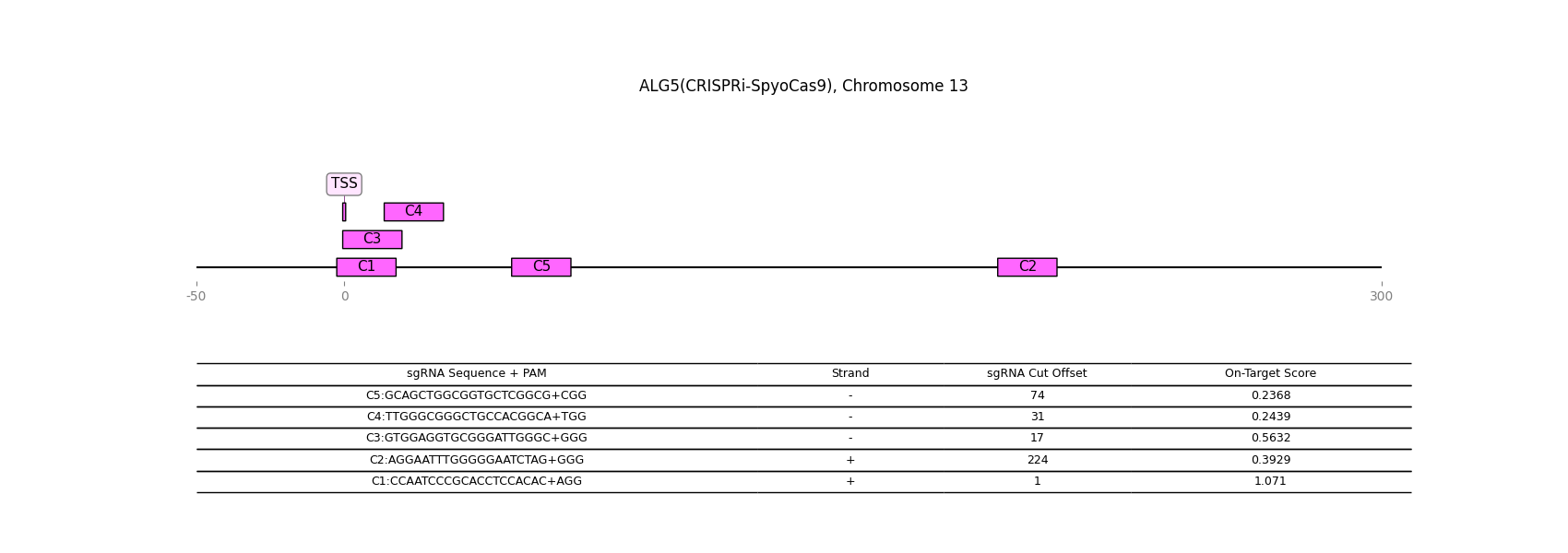
CRISPR-inactivation
Transcription factor-gene relationship (details at glycoTF page)
Top 10 TFs
| TF | Score |
|---|---|
| TCF25 | 1.175115 |
| SON | 1.147646 |
| RBM39 | 1.142036 |
| XRCC5 | 1.139874 |
| HNRNPK | 1.139126 |
| UBE2I | 1.137317 |
| SSU72 | 1.123587 |
| SRSF3 | 1.120158 |
| PCBP1 | 1.110878 |
| PCBP2 | 1.107422 |
Licensing: CC BY 4.0. You are fee to copy, redistribute, remix, transform and build upon all material, except for textbook figures from the Essentials.